Key Market Insights
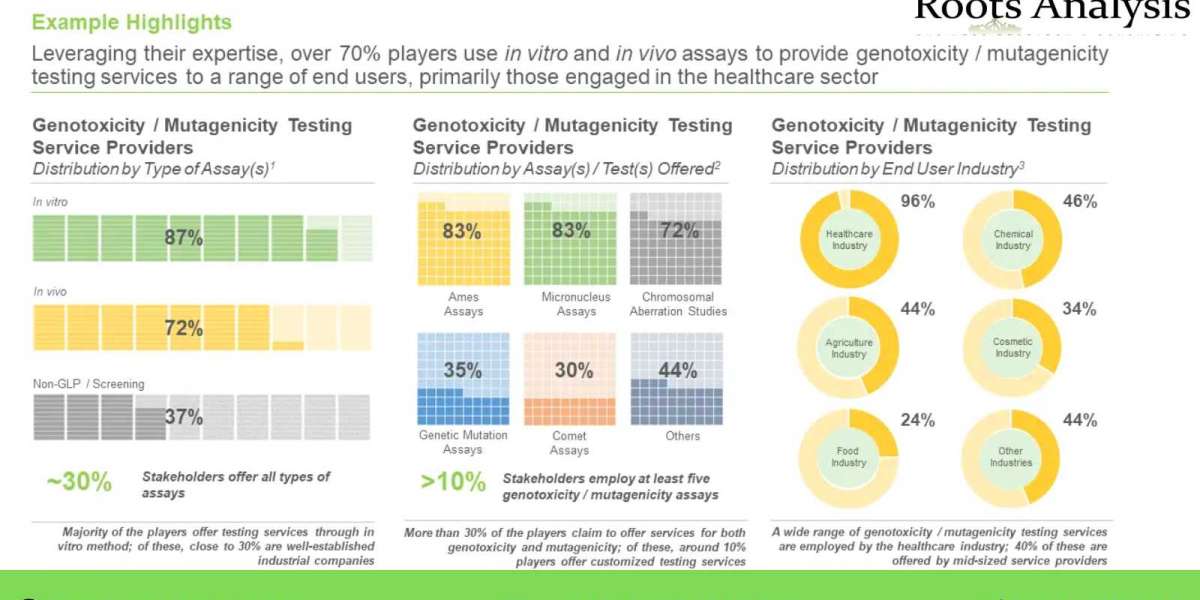
- Presently, over 80 players claim to have the necessary capabilities to offer services related to genotoxicity / mutagenicity testing, which can be used for the safety assessment of various components
- The current market landscape features the presence of both established and emerging players, having the capability to provide genotoxicity / mutagenicity testing services
- In pursuit of gaining a competitive edge, service providers claim to be steadily expanding their existing capabilities to enhance their expertise in the genotoxicity / mutagenicity testing related domain portfolios
- Over the past few years, the field has witnessed notable efforts in terms of partnerships and collaborations for increasing genotoxicity / mutagenicity testing portfolio; this reflects the rising interest of stakeholders in this domain
- A detailed review various patents filed / granted in the field of genotoxicity and mutagenicity, based on relevant parameters, such as type of patent, publication year, geographical region, CPC symbols, leading players and type of applicant
- The overall opportunity is anticipated to grow at the CAGR of 7%; it is likely to be well distributed across different type of assays
Table of Contents
- PREFACE
1.1. Scope of the Report
1.2. Market Segmentation
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Detrimental Effects of Genotoxins / Mutagens
3.3. Mechanism of Genotoxicity / Mutagenicity
3.4. Techniques Employed for Genotoxicity / Mutagenicity Testing
3.5. Applications of Genotoxicity / Mutagenicity
3.6. Recent Developments and Future Perspectives
- MARKET LANDSCAPE
4.1. Genotoxicity / Mutagenicity Testing Service Providers: Overall Market Landscape
4.2. Genotoxicity / Mutagenicity Testing: Service Provider Landscape
- BENCHMARKING ANALYSIS
5.1. Scope and Methodology
5.2. Assumptions and Key Parameters
5.3. Parameters Scoring
5.4. Benchmarking Analysis: Players based in North America
5.5. Benchmarking Analysis: Players based in Europe
5.6. Benchmarking Analysis: Players based in Asia-Pacific
- COMPANY PROFILES
6.1. Charles River Laboratories
6.1.1. Company Overview
6.1.2. Financial Information
6.1.3. Service Portfolio
6.1.4. Recent Developments and Future Outlook
6.2. LabCorp
6.3. Syngene
6.4. Aurigene Pharmaceutical Services
6.5. Sai Life Sciences
6.6. LSIM Safety Institute
6.7. GLR Laboratories
- PUBLICATION BENCHMARKING ANALYSIS
7.1. Scope and Methodology
7.2. Assumptions and Key Parameters
7.3. Analysis by Year of Publication
7.4. Analysis by Type of Article
7.5. Popular Journals: Analysis by Number of Publications
7.6. Popular Publishers: Analysis by Number of Publications
7.7. Analysis by Journal Impact Factor
7.8. Popular Journals: Analysis by Journal Impact Factor
7.9. Publication Timeline Analysis
7.10. Publication Benchmarking Methodology
7.11. Publication Benchmarking Analysis
7.12. List of Top 10 Publications
- GRANTS ANALYSIS
8.1. Scope and Methodology
8.2. Assumptions and Key Parameters
8.3. Analysis by Year of Grant Award
8.4. Analysis by Amount Awarded
8.5. Analysis by Funding Institute Center
8.6. Analysis by Administering Institute Center
8.7. Analysis by Support Period
8.8. Popular Recipient Organizations: Analysis by Number of Grants
8.9. Analysis by Type of Recipient Organization
8.10. Analysis by Purpose of Grant
8.11. Analysis by Study Section
8.12. Popular NIH Departments: Analysis by Number of Grants
8.13. Analysis by Administering Institute Center and Support Period
8.14. Analysis by Type of Grant Application
8.15. Analysis by Grant Activity Code
8.16. Prominent Program Officers: Analysis by Number of Grants
8.17. Analysis by Location of Recipient Organization
- PARTNERSHIPS AND COLLABORATIONS
9.1. Partnership Models
9.2. Assumptions and Key Parameters
9.3. Genotoxicity / Mutagenicity Testing: List of Partnerships and Collaborations
- PATENT ANALYSIS
10.1. Scope and Methodology
10.2. Assumptions and Key Parameters
10.3. Genotoxicity and Mutagenicity Testing: List of Patents
10.4. Analysis by Type of Patent
10.5. Analysis by Patent Publication Year
10.6. Analysis by Annual Number of Granted Patents
10.7. Analysis by Geographical Location
10.8. Analysis by Jurisdiction
10.9. Analysis by CPC Symbols
10.10. Analysis by Type of Applicant
10.11. Analysis by Patent Age
10.12. Word Cloud: Emerging Focus Areas
10.13. Leading Industry Players: Analysis by Number of Patents
10.14. Leading Non-Industry Players: Analysis by Number of Patents
10.15. Leading Individual Assignees: Analysis by Number of Patents
10.16. Leading Players: Benchmarking by Patent Characterization (CPC Symbols)
10.17. Patent Valuation: Analysis Methodology and Parameters
- MARKET SIZING AND OPPORTUNITY ANALYSIS
11.1. Forecast Methodology and Key Assumptions
11.2. Global Genotoxicity / Mutagenicity Testing Services Market, 2023-2035
11.2.2. Genotoxicity / Mutagenicity Testing Services Market, 2023-2035: Analysis by Assay / Test Offered
11.2.3. Genotoxicity / Mutagenicity Testing Services Market, 2023-2035: Analysis by End User Industry
11.2.4. Genotoxicity / Mutagenicity Testing Services Market, 2023-2035: Analysis by Geographical Region
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/genotoxicity-testing-services-market.html
You may also be interested in the following titles:
Decentralized Clinical Trials / Virtual Clinical Trials Market |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415